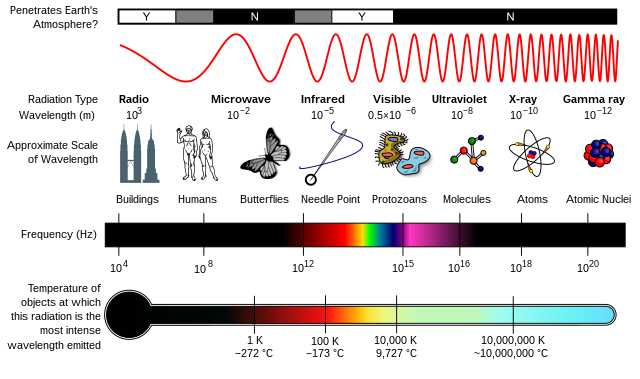
The electromagnetic spectrum is a continuous range of wavelengths. The types of radiation that occur in different parts of the spectrum have different uses and dangers — depending on their wavelength and frequency.
Throughout most of the electromagnetic spectrum, spectroscopy can be used to separate waves of different frequencies, producing a spectrum of the constituent frequencies. Spectroscopy is used to study the interactions of electromagnetic waves with matter.
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 1025 hertz, corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. This frequency range is divided into separate bands, and the electromagnetic waves within each frequency band are called by different names; beginning at the low frequency (long wavelength) end of the spectrum these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays at the high-frequency (short wavelength) end. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. There is no known limit for long and short wavelengths. Extreme ultraviolet, soft X-rays, hard X-rays and gamma rays are classified as ionizing radiation because their photons have enough energy to ionize atoms, causing chemical reactions. Exposure to ionizing radiation can be a health hazard, causing radiation sickness, DNA damage and cancer. Radiation of visible light and longer wavelengths are classified as nonionizing radiation because they have insufficient energy to cause these effects.
The order of electromagnetic waves in the spectrum is shown in the table.
| Energy | Frequency | Wavelength | Radiation type | Typical use |
|---|---|---|---|---|
| Lowest | Lowest | Longest | Radio waves | Television signals |
| Microwaves | Cooking, mobile phones | |||
| Infrared | Optical fiber communication | |||
| Visible light | Seeing | |||
| Ultraviolet (UV) | Detecting forged bank notes | |||
| X-rays | Medical images of bones | |||
| Highest | Highest | Shortest | Gamma radiation | Killing cancer cells |
Radio waves have the lowest frequencies and longest wavelengths, while gamma waves have the highest frequencies and shortest wavelengths.
All of these waves travel at the same speed in free space, which is the speed of light or about 300,000,000 m/s (meters per second).
Uses of electromagnetic radiation
| Electromagnetic radiation | Uses | Ionizing or Non-ionizing |
|---|---|---|
| Radio waves |
broadcasting and communications — longer wavelengths mean they travel further in the earth's atmosphere, reflecting off hills and the upper atmosphere. |
Non-ionizing |
| Microwaves |
Cooking food — microwaves are absorbed by water molecules causing them to vibrate (heat up). Satellite transmissions — their wavelengths penetrate earth's atmosphere. |
Non-ionizing |
| Infrared (IR) |
Heater and night vision equipment — all objects, including people, give out infrared rays which can be detected even at night. It's also used for television remote controls. |
Non-ionizing |
| Visible light |
Human vision, photography and optical fibers — it's the only part of the spectrum that humans can see. |
Non-ionizing |
| Ultraviolet (UV) |
Fluorescent lamps — they have chemicals inside them which absorb ultraviolet rays and convert the energy to visible light. |
Higher-energy / Extreme ultraviolet (EUV) are ionizing radiation otherwise Ultraviolet if non-ionizing. |
| X-rays |
Medical equipment — they enable us to see the internal structure of objects and materials by passing through some substances (e.g., body tissue) but being absorbed by others (e.g., bone). |
Ionizing |
| Gamma rays |
Sterilizing food and medical equipment — they are highly penetrative and can kill. |
Ionizing |
Hazards of electromagnetic radiation
Over-exposure to certain types of electromagnetic radiation can be harmful. The higher the frequency of the radiation, the more damage it is likely to cause to the body:
- microwaves cause internal heating of body tissues
- infrared radiation is felt as heat and causes skin to burn
- X-rays damage cells causing mutations (which may lead to cancer) and cell death - this is why doctors and dentists stand behind protective screens when taking lots of X-rays
- gamma rays also damage cells causing mutations and cell death
Microwave radiation
Microwave radiation can be used to transmit signals, such as those for mobile phone calls. Microwave transmitters and receivers on buildings and masts communicate with the mobile phones in their range. There is concern that microwave radiation from mobile phones and masts may be harmful to health.
Ultraviolet radiation
Ultraviolet radiation (UV) - is found naturally in sunlight. We cannot see or feel ultraviolet radiation, but our skin responds to UV exposure by turning darker over time. This is called a sun tan. This happens as our bodies attempt to reduce the amount of ultraviolet radiation reaching deeper skin tissues.
Darker skins absorb more ultraviolet light, so less ultraviolet radiation reaches the deeper tissues. This is important, because ultraviolet radiation can cause cells to become cancerous.
People should wear UV blocking sunscreen on sunny days to avoid skin cancer. Overexposure of the eyes to ultraviolet radiation can cause blindness, so we should wear hats and sunglasses on sunny days.
The three main types of ultraviolet radiation and some of their effects
| Type | Frequency | Hazard |
|---|---|---|
| UV-C | High | Causes severe damage to cells |
| UV-B | Medium | Causes severe sunburn and damage to cells |
| UV-A | Low | Weaker effects than UV-B |
Glossary
- Dispersion
- Spreading out of the different wavelengths of light, caused by refraction of light as it passes through a prism.
- Electromagnetic radiation
- Energy travelling as waves in the form of changing electrical and magnetic fields.
- Frequency
- The number of waves produced each second. The unit of frequency is hertz (Hz).
- Ionizing radiation
- Radiation that consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Ionizing radiation is used in a wide variety of fields such as medicine, nuclear power, research, and industrial manufacturing, but presents a health hazard if proper measures against excessive exposure are not taken. Exposure to ionizing radiation causes cell damage to living tissue and organ damage. In high acute doses, it will result in radiation burns and radiation sickness, and lower-level doses over a protracted time can cause cancer.
- Non-ionizing radiation
- A type of electromagnetic radiation (EMR) that does not carry enough energy per quantum (photon energy) to ionize atoms or molecules — that is, to completely remove an electron from an atom or molecule.
- Radiation
- Energy carried by particles from a radioactive substance or spreading out from a source.
- Refraction
- Process by which a wave changes speed and sometimes direction upon entering a denser or less dense medium, e.g., a light ray changes direction when refracted by a lens.
- Ultraviolet light
- Electromagnetic radiation with a greater frequency than visible light but less than X-rays. Humans cannot see it, but it can damage eyes and skin in high doses.
- Ultraviolet radiation
- Electromagnetic radiation with a frequency between that of visible light and X-rays.
- Wavelength
- he length of a single wave, measured from one wave peak to the next.
- White light
- Light that is a mixture of all the frequencies of visible light, so it appears white.